As a follow-up to my radiation treatment for prostate cancer, that I reported here as: Medical fun and games, I recently underwent a PET scan, to check that all is well.
When I first heard of them I imagined that a PET scan was a more generic all-encompassing version of a CAT scan - perhaps one involving dogs and rabbits; or even goldfish?
Perhaps you know the story:
A man's dog goes to sleep and won't wake up.
So, he takes it to the veterinarian.
Harriet, the vet, tells him: 'Your dog's dead.'
'Oh no,' says the man, 'he's just a very heavy sleeper.'
'No,' insists Harriet, 'he's dead!'
But the man won't accept it, insisting the dog's just sleeping.
'So,' Harriet says: 'Does your dog like cats?'
'No.' says the man: 'He hates cats - goes berserk at the mere sight of one.'
'Ok!' says Harriet.So she goes out to the boarding cages and brings back a kitten. She then presents the kitten to the recumbent dog's nose - nothing.
Then she encourages the kitten to walk all over the dog - still nothing!
So Harriet shakes the dog shouting: 'Wake up!' Then repeats the process.
'Enough! Stop!' cries the man: 'I told you, he's just a heavy sleeper. I'll just take him home and wait for him to wake up. What do I owe you?'
Harriet says: '$1,050'
The man is outraged: '$1,050 for treating a dead dog! The bloody dog's dead!'
'That's right,' says Harriet: 'That's $50 for the consultation and $1,000 for the CAT scan."
But no. The acronym: PET refers to 'positron emission tomography', as opposed to 'computed axial tomography' - CAT. And, as I'm now becoming very familiar with, it involves lying on a table (or couch) that is fed in-and-out, often multiple times, through the donut-like ring of a rotating scanner.
 A CT Scanner - from Wikipedia Creative Commons
A CT Scanner - from Wikipedia Creative Commons
The same device is able to perform both CAT and PET scans and both of these scans involve, potentially harmful, ionising radiation with strict exposure limitations that require operators to vacate the room when the machine is running.
In this respect, they differ from an MRI (magnetic resonance imaging) scanner, that involves passing through a similar tunnel of rotating detectors. But in the MRI case, the image is derived using a very strong enveloping magnetic field, and images tissue using photons at radio frequencies, that are harmless (except that the magnetic flux may injure people with certain metallic implants, including heart pacemakers) and so they have less exposure limitations, allowing much longer scan times.
A CAT or CT scanner emits X-rays. These are detected at 180 degrees, opposite, on the rotating ring, in multiple slices, as the patient-table is advanced thought its tunnel. Thus providing a three dimensional X-ray map of the interior of the patient's body. The scan is usually repeated before the patient moves: the first scan without a revealing dye and a second after a dye has been injected.
A PET scan does not require external x-rays as it involves an injected radioactive isotope that, as the name implies, involves positrons.
But, how could positrons be emitted I wondered? As I remembered, positrons are antimatter. So they are immediately annihilated if they meet an electron. How do they manage to escape the body, through a vast cloud of electrons surrounding all those nearby atoms, to reach a detector?
The answer is: they don't. No sooner are they released, by the conversion of a proton to a neutron, than they are annihilated, typically by one of the source atom's own electrons.
As a result of this annihilation of matter, energy is released (remember Einstein: E = mc²) in the form of a photon pair (very high energy electro-magnetic radiation) polarised at 180° relative to its twin.
Most of us are familiar with polarised photons, having owned polarised sun glasses; or from those polarised glasses that they hand out at recent 3D movies.
Positron annihilation photons also have a distinct frequency (colour) in the gamma spectrum; equivalent to 511 Kev (thousand electron volts). This is a much higher frequency (shorter wavelength) than visible light and shorter even than the ordinary, 'soft' x-rays, used by your dentist or your doctor to see a broken bone.
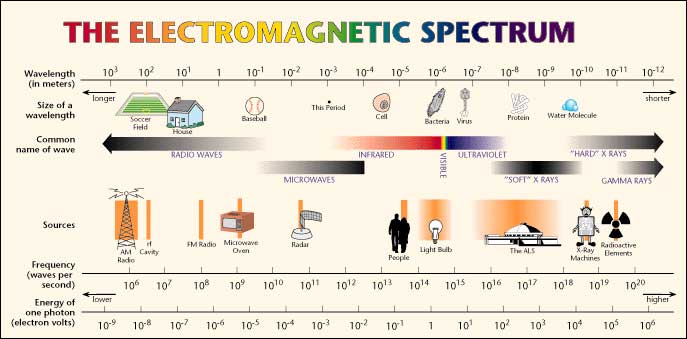
So, the term 'positron emission' relates not to the positrons themselves but to the annihilation photons emitted.
Like gamma rays from space, most of these photons pass through the body tissue unimpeded. Specialist detectors, similar (in principle) to those used in the camera in your phone, convert the photons to digital pulses (encoding images, as you do each time you snap a 'selfie'), to be fed into the tomography (slice-photographing) computer.
So, now I understood the name - nothing to do with cats or dogs.
But, you may well ask, how are these positrons, and thus the photons, emitted? And what's the point of scanning them anyway?
Well, as you no doubt know, the 30 to 40 trillion cells in an adult human body obtain their energy, to persist (continue living) and to divide (multiply), from blood sugars (glucose).
Rapidly multiplying cancer cells are unusually hungry for blood sugar. So, if one of these sugars is compounded with a positron emitter, and then injected into a patient's blood-stream, after half an hour, or so, the cancer cells will absorb more than the normal cells and 'light up', emitting more positron-annihilation photons (gamma rays).
Yet, ionising radiation is dangerous and producing it is not the sort of thing one wants to go on for very long inside you. It will begin killing cells and/or damaging DNA in the vicinity, quite quickly.
As Alice muses to herself, after falling down the Rabbit Hole to Wonderland: "If you drink much from a bottle marked 'poison' it is certain to disagree with you sooner or later."
So choosing a positron embitter with a short half-life is desirable. But not so short that it doesn't last long enough to be incorporate it into a glucose molecule, in a laboratory process, and then for the resultant product to be transported: to be injected into a patient; and then to accumulate at a cancer site, prior to scanning.
There are at least 16 elements that have isotopes that that decay by positron emission to other elements. This happens when one or more of protons in their nucleus become neutrons (emitting a positron in the process). Among those having useful half-lives, are: carbon-11; nitrogen-13; oxygen-15; fluorine-18; and sodium-22.
Each of these is used, in some context, in positron emission tomography. Yet, on Earth, none of these exist in significant quantities in nature, except briefly, as a result of lightening. They can be made in a nuclear reactor or in an atomic explosion but are not easily collected.
So, the usual method of converting one element to another is by bombarding the nucleus of the source element with a beam of protons that have been accelerated in a cyclotron or accelerated using a linear particle accelerator.
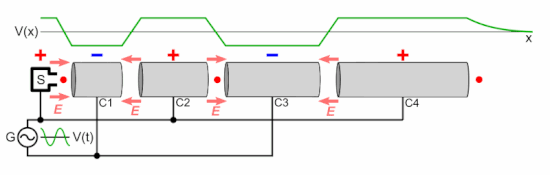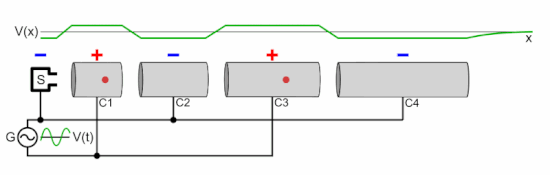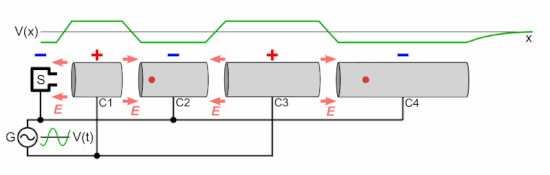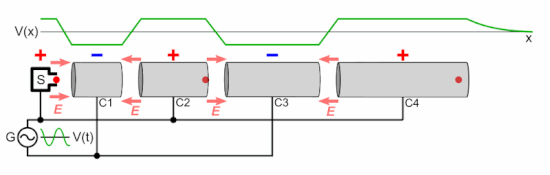 Chetvorno, CC0, via Wikimedia Commons
Chetvorno, CC0, via Wikimedia Commons
In a linear accelerator, adding energy to protons is achieved by a sequence of ever lengthening tubular electrodes, excited to alternate in polarity, pulling then pushing (opposite polarities attract equivalents repel), at microwave frequency. At each electrode the proton beam absorbs more energy. This energy is derived from a magnetron and an associated wave guide (like the active device in a microwave oven). In a cyclotron this is done in a circular racetrack.
Thus, the dream of the alchemists, of turning one element into another, is now carried out every day in many places around the world.

But turning lead into gold is still a big stretch. And most of the new elements, thus created anew, are unstable (radioactive) isotopes.
The half-life is the time it takes for half the unstable atoms to have decayed to another, usually stable, element. It does not mean, as some seem to imagine, that an unstable isotope is now harmless. If unstable isotopes disappeared after their half-life, there would be no uranium-235 (half-life 703.8 million years) at all left on Earth since it's well over 4 billion years since the supernova in which it was created. It just means that, if it were left unused, in 704 million years it would be down to half as much as there is now. So we'd better dig it up and use it pronto?
In the case of sodium-22, the half-life is 2.6 years, not something one would like a lot of inside one. So its medical uses are limited.
But the half-life can't be too short. After the creation of a short-half-life isotope the the clock is ticking. The newly converted atoms have to be incorporated into an organic carrier-molecule as fast as possible.
This can't be done first, as the proton beam would destroy the desired molecule.
For several (carbon; nitrogen; and oxygen) the half life is so short that the particle accelerator has to be on-site. Fortunately, fluorine-18 (half-life of just under 110 minutes) is in the Goldilocks zone - not too long not too short - making it the most commonly used positron emitter used in PET scans. Fluorine-18, conveniently, decays to oxygen-18 (heavy oxygen), that's both stable and harmless. Two of the newly created oxygen atoms typically pick-up a carbon atom to become carbon dioxide. Or each new oxygen atom picks up two hydrogen atoms, to become water, that's soon excreted.
Once created, the fluorine-18 is incorporated into a glucose molecule, using conventional organic chemistry, to become fludeoxyglucose (FDG) or a similar glucose molecule, before injection into the patient. Obviously this substance is radioactive, so it's held in a radiation-proof-flask, with the nurse stationed behind a shield, manipulating a plunger that ejects the flask contents into a saline carrier and then into a cannular in the patient's arm.
The patient then rests for at least 20 minutes to allow the glucose to be absorbed by any hungry, rapidly-dividing mutant cells.
Several images are then compiled, typically two CT scans and the PET scan, during which the patient is asked not to move. This is not too arduous as the whole scanning process takes about 20 minutes and is non-invasive, except for the dye injection, pumped in through the same cannular that was used for earlier the FDG injection.
I find the dye quite pleasant. It warms my body like a 'wee dram' (or two) of Drambuie. I could go for that again. Although, the friend-of-a-friend reported feeling chilled. Perhaps it had just come out of a fridge?
For several hours after receiving FDG one is radioactive - emitting gamma radiation - and hospital staff take precautions. But with a half-life of just under two hours the radiation is back down to background levels in about 15 hours.
In the meantime, don't go top bed with a pregnant woman or get a woman pregnant. Unfortunately, that's no longer a big risk for me.
The bottom line in all this is: that in the course of my lifetime all this - including the transmutation of elements and the computerisation of medicine has become possible. Now tens of thousands are cured, who would otherwise have died earlier and more unpleasantly. And yet again, just as I needed the technology, there it was.
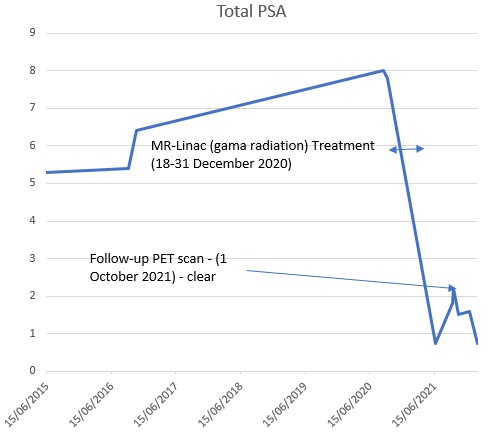
On this occasion, it seems that the small rise in total PSA (prostate specific antigen) was a temporary 'glitch' during recovery as no nasty mutant cells were found lurking. Yet it wasn't wasted on me.
It's all so fascinating.