Approaches to Electricity Storage
Storage Batteries (accumulators)
We are all familiar with batteries (accumulators). There have been huge advances in battery technology in my lifetime that have made electricity storage on a megawatt-hour (MWh) scale practical.
Accumulators, also called 'secondary cells', store electricity by converting it to chemical energy employing a reversible chemical reaction that enables them to be charged and discharged repeatedly.
Although small batteries (D; AA and AAA cells for example) are often packaged alike, to facilitate interchangeably, accumulators should not be confused with one-use 'primary cells', that employ an irreversible electrochemical reaction that consumes or depletes the chemicals employed.
In addition to high energy efficiency (very low charge and/or discharge losses) an ideal storage battery would be constructed from plentiful; non-toxic chemicals; and would be light weight to enable its use in vehicles and hand-held devices. It would work in any climate and safely store chemical energy at a higher density than any other storage.
Obviously, the chemical reaction employed must be reversible and be stable over time. Cycling should be possible many thousands of times over, before the storage qualities are significantly degraded. And recharging should be as fast as possible.
For more than a century our best rechargeable battery was the lead-acid accumulator, like that used to start a conventional car; truck; or bus.
Then, in the last decade of the 20th century, new materials based on rare-earths offered new avenues of research. Commercial nickel metal-hydride (NiMH) batteries, became a reality. Nickel is less dense than lead and the new batteries packed twice as much energy per kilogram as lead-acid cells. As an added bonus they could be small or large and had no messy liquid acid or fumes. This improvement opened the way to many new types of hand tools and electric vehicles.
For many years lithium shimmered as the 'Holy Grail' of battery technology. Lithium is the lightest metal and the least dense solid element. It's also highly reactive, with the greatest electrochemical potential and energy-to-weight ratio of any element in the periodic table.
But lithium metal is so reactive that it spontaneously bursts into flame and early attempts to build a safe and practical battery ended in tears. Nevertheless, work went on, involving many improvements until, in 1985, Akira Yoshino assembled the first practical rechargeable lithium-ion battery at the Kawasaki Laboratory of the Asahi Kasei Corp in Japan. His new battery avoided using lithium metal directly, making it less unstable, yet provided another doubling in energy density over NiMH.
With volume, the battery prices quickly fell, so that new opportunities and markets, like little drones and more complex telephones, proliferated. The trouble is that Li-ion batteries are still rather dangerous. Higher current energy density comes with a risk, as Boeing discovered on their new 787; as did UPS when a battery fire brought one of their planes down. Samsung too had to withdraw their Samsung Galaxy Note 7 due to higher density batteries catching fire.
Further increase in chemical energy density is likely to make accumulators even more dangerous, equivalent to a high explosive, like TNT.
The electric car maker Tesla is well aware of the dangers inherent in the lithium-ion (Li-ion) batteries it uses. At the moment the Tesla battery has about the same energy as 75 kg of TNT. To obviate the risk of a single explosion the Tesla 'battery' is not a single unit but consists of over seven thousand individual Li-ion cells, similar to the familiar 'D' cell, packed into 16 separate modules, that are distributed across a wide pan below the car.
Despite the well-published failures, an attractive feature of Li-ion technology, compared to other very high energy batteries, is its relative safety. So, most of us walk around happily with a small one in our mobile phone.
Some advocates of all-electric cars promote them as grid storage. But this is problematic as they could also have the effect of contributing to the demand- supply mismatch. Cars are mostly driven during the day, at morning and evening peak-hour, and charged in the middle of the day and at night. Daytime charging may match peak solar but drivers do not stop to charge when the wind is blowing or avoid charging when it is dark and there is no wind.
Stationary batteries are designed to do just this and are a much better option than those being driven about. Thus, many homes with rooftop solar panels, now also have a storage battery to smooth the peaks and troughs in the electricity generated as the solar incidence on their panels varies, often minute to minute; hour to hour. And on a grid supply scale, South Australia, that is saturated with variable wind energy, has installed several mega-batteries, to cope with mismatched demand and supply peaks.
Continuing improvement in batteries can be expected and there are other options beyond lithium for stationary batteries, where energy density is less important that overall efficiency (minimal energy lost during a cycle) and capital cost (per MWh stored).
Here are some features of present generation of, advanced, high-energy-density, batteries:
| Nickel metal-hydride (NiMH) Energy density Specific power Charge/discharge efficiency Self-discharge rate Low self-discharge types: Cycle durability |
140–300 Wh/L 250–1,000 W/kg 66%-92% 13.9–70.6% (per month) 1.3–2.9% (per month) at 20 °C 180–2000 cycles |
Lithium-ion Energy density Specific power Charge/discharge efficiency Self-discharge rate Cycle durability |
250–676 Wh/L ~250-~340 W/kg 80–90% 8% (per month) at 21 °C 400–1200 cycles |
Source: Wikipedia: NiMH - Lithium-ion
Hydrogen
That electricity can be used to disassociate water, into hydrogen and oxygen, was one of the fist observations arising out of the discovery of electrical currents at the turn of the 18th century. Electrolysis was described, and named, by Michael Faraday, at that time Sir Humphry Davy's research assistant responsible for demonstrations of scientific discoveries to the educated classes in England. Later Faraday would make ground-breaking discoveries himself including his 'first and second laws of electrolysis' in 1833. Both the Faraday Cage, that protects high-voltage workers from death, and the Farad, the unit of electrical capacitance, are named after him.
As a result, hydrogen was soon seen to be a possible means of capturing electrical energy. As home and then in High School physics we did it for ourselves.
For example, in 1874 Jules Verne had a similar vision but misunderstood electricity. Believing it to be like gas, a source of the energy in itself:
The Mysterious IslandJules Verne 1874 “Yes, but water decomposed into its primitive elements,” replied Cyrus Harding, “and decomposed doubtless, by electricity, which will then have become a powerful and manageable force, for all great discoveries, by some inexplicable laws, appear to agree and become complete at the same time. Yes, my friends, I believe that water will one day be employed as fuel, that hydrogen and oxygen which constitute it, used singly or together, will furnish an inexhaustible source of heat and light, of an intensity of which coal is not capable. Some day the coalrooms of steamers and the tenders of locomotives will, instead of coal, be stored with these two condensed gases, which will burn in the furnaces with enormous calorific power. There is, therefore, nothing to fear. As long as the earth is inhabited it will supply the wants of its inhabitants, and there will be no want of either light or heat as long as the productions of the vegetable, mineral or animal kingdoms do not fail us. I believe, then, that when the deposits of coal are exhausted we shall heat and warm ourselves with water. Water will be the coal of the future.” |
Then in 1923, scientist and polymath, J B S Haldane, rectified this rather serious shortcoming: an inexhaustible source of heat and light; by proposing a network of electricity-generating windmills to produce hydrogen for distribution in Britain.
Hydrogen was, by then, already being piped around British and Australian cities (also British at the time) from gasworks, that manufactured producer gas (a mixture of hydrogen and carbon monoxide) from coal and steam. And the house in which I'm writing this was once lit by gas; heated by gas (still is); cooking was by gas (some still is) and the hot water was by gas (ditto). So, when I go to the side of the house, I still see a slope, and drop pipes below upward pipes, on the original gas plumbing. These were designed to capture entrained water vapour that condensed out. So, occasionally, if the gas petered-out, the plug at the bottom of the run would be removed to drain water from the system.
Yet these are no longer necessary, the gas no longer contains water vapour (or free hydrogen) - the gasworks are all gone to be superseded by natural gas - mostly methane.
So, given that hydrogen, from electrolysis, has been fêted for so long, why hasn't it happened yet?
There are several reasons:
Cost, handling, safety and competitiveness; when used as a transport fuel:
Hydrogen is a lot cheaper to make in the old way, than by electrolysis. Commercial hydrogen is made by applying steam and extra heat to a hydrocarbon like petroleum; or natural gas; or coal. This generates a lot of carbon dioxide as a by-product (and wastes energy, compared to burning the hydrocarbon or coal directly).
Yet, even this relatively inexpensive, anything but green, hydrogen, has not taken off as a transport fuel.
This is partly due to distribution and storage issues, compounded by some unique safety concerns.
Although hydrogen is very light and liquid hydrogen has three times the energy per kg of LPG (it's good as a rocked fuel) it takes around three litres of liquid hydrogen to provide the same energy as a litre of LPG. And with a boiling point of −252.9 °C (−423.2 °F) it's much harder to compress and store than LPG (-42°C or -44°F) that is easily compressed and transported as liquid at normal temperatures.
To be used as a transport fuel, hydrogen must either be compressed, or converted to a metal hydride.
Compressed to 50 atmospheres pressure, as used in cars and buses, hydrogen has less than a 50th of the energy per litre than petroleum or natural gas. This requires much larger and more expensive tanks and dramatically reduces the practical range, compared to LPG powered vehicles.
So, why destroy a perfectly good transport fuel like LPG; or natural gas; or even coal; to make an inferior one?
And when it comes to hydrogen fuelled (using a fuel cell) electric vehicles: why not simply use the electricity directly?
Energy efficiency:
To be useful, as an electricity storage means, very little of the energy should be lost in recovering the electricity. Yet hydrogen is very poor in this regard.
Conventional electrolysis just electrodes in water, as we did as kids, is very inefficient, wasting up to half of the electrical energy, but new polymer electrolyte membrane (PEM) electrolysis is much more efficient, at around 85% and possibly higher (15% lost to start with). But that's just half the story.
To get the electricity back there are two approaches: the hydrogen can be used to power a heat engine, to spin a dynamo or alternator, or the hydrogen can be reacted with oxygen in a fuel cell, recovering the water destroyed during electrolysis.
The former is very wasteful, as heat engines lose up to 70% of the energy expended.
Hydrogen fuel cells are more efficient but still convert 60% to 70% of the energy to heat (rather than electricity).
This is fine if you can use the heat, for example: to heat domestic or industrial water, but a terrible way to recover the electricity originally invested.
If we assume 15% of the energy is lost during electrolysis and at least 40% is lost converting the hydrogen back to electricity, then the best equivalent charge/discharge efficiency we could expect would be 35%. This compares to a lithium-ion battery that achieves 80–90% (see above). Further, the equipment involved is far more complex and costly than an equivalent modern battery.
Hydrogen as a transmission medium:
Some have suggested, like Haldane in 1923, that electrolysis could be an option for solar or wind power stations in remote locations, like Central Australia. The hydrogen would be then be piped to where the electricity is needed. But this raises several more issues. Pipelines require pumping stations and these require energy, usually drawn from the gas or liquid being piped. The overall losses, together with capital servicing costs, would need to be less than possible alternatives like: very high voltage DC transmission; or even batteries on a train (boat; trucks; - you name it).
Yet there is a scenario in which electrolysis of water makes sense; and it goes back to J B S Haldane. If electrically-disassociated-hydrogen, from wind; or PV solar generators; was added to existing gas distribution networks, as Haldane envisaged, it would transmit the energy to down-stream gas users and lower the overall release of carbon, when the gas is burnt.
This would require significant volumes of water (making hydrogen from water obviously requires water). And around the same volume of water destroyed at the source is recreated at the end. This would limit the practicality in some locations, like arid Central Australia, but coastal windfarms suggest themselves. And some larger energy markets, that already have desalination plants, might benefit from some additional water.
But as a storage method for electricity, hydrogen is a non-starter - wasteful of energy and uncompetitive with several other options.
Again, a paper, that is much more erudite than mine, convincingly confirms that converting electricity to hydrogen and then back to electricity in vehicles makes no sense. Batteries do it a great deal more efficiently. Click here to read Professor Bossel's analysis...
The same applies to other applications, like load-shedding, as there are several other methods of storing electricity that are vastly superior to employing hydrogen.
Pumped-Storage Hydropower (PSH)
The Australian Federal Government is pinning its hopes on pumped-storage.
Until recently the only commercial large scale (terawatt) method for storing energy was pumped-storage, associated with hydroelectric schemes. The best of these loses 20 to 30% of the energy in pumping water up hill and then letting it run back through the turbines. While the energy efficiency is less than the latest batteries, in the long term, pumped-storage may be more cost-effective.
Pumped storage is primarily used for providing extra generation when consumer demand is high; or pumping when there is a long spell of excessive generation form wind or solar; not for absorbing wildly fluctuating generation from wind or solar farms, where batteries are now proving effective.
In Australia present hydro-generation is far too small and unresponsive to compliment a large-scale contribution of solar energy. In 2021 the capacity of the Snowy Mountains Hydroelectric Scheme is only 7.8GW. Present pumped storage capability is presently 0.6 GW, a tiny fraction of this. This will be expanded with the addition of 27km of tunnels and building a new underground power station, by a further 2 GW, when the present Snowy 2.0 project is completed in 2026.
To put this into context, total installed electricity capacity in the National Electricity Market (NEM) in 2021 is 52.5 GW. So, after completion, Snowy 2.0 will contribute less than 5%. This is significant at present but not if the mix of highly variable renewable rises to become a larger part of the generation mix. By comparison the installed capacity of Eraring, just one of of five coal-fired power-stations in New South Wales, is 2.8 GW.
Back in the day, when Josh Frydenberg (now Treasurer) was Federal Energy Minister, he proposed a pumped hydro storage facility at Cultana on the Eyre Peninsula that could protect South Australia from power shortages that lead to load-shedding blackouts. And announced that work is underway to make such a facility a reality.
"The Cultana defence site has pipelines, transmission lines and roads connected to it... It might only take a couple of years to complete," he assured South Australians.
He went on to point out that: "97% of {world} energy storage was in pumped hydro. "It’s been underdone in Australia,” he said: “We have now tasked the Australian Renewable Energy Agency and the Clean Energy Finance Corporation in a new funding round for large-scale storage, including pumped hydro.”
ANU Professor Andrew Blakers (Engineering) supported the Minister's proposal.
"The possibilities are almost unlimited," Prof Blakers is reported as telling the Adelaide Advertiser (or was it a press release?): "because you can build as many facilities as you like... We’re looking for a decent volume, and a big height differential from the upper to the lower. Ideally 400 to 500 metres," the good Prof enthused.
"It would be largely trouble free, using well proven hydro generation technology, and last for in excess of 50 years, as opposed to batteries which, very optimistically, even if improved, would last for a tenth of that time."
"You would look typically at 300 MW and a 10-hectare reservoir... If you want more, you just make more - four or five or six.”
"This form of hydro doesn’t need a river. It works by pumping water from a lower reservoir to a higher one when energy is cheap, then letting the water run down again through a turbine when it’s expensive... A standard model would cost up to $500 million, but would last 50 years and be able to generate 300 megawatts."
Meanwhile, Josh reminded us, that he and the Prime Minister had already announced a $2 billion expansion of the Snowy Mountains hydro scheme to address energy security: "...preventing power shortages in eastern states... The first major expansion of Snowy Hydro scheme since construction completed in 1974."
In the event, in 2018 the Cultana Pumped Hydro Energy Storage Project proceeded to a detailed engineering and financial pre-feasibility analysis (Phase 2 of the project). This found environmental; unforeseen capital cost; and competition (from alternative solutions) issues that rendered this project non-viable.
You can read the Energy Australia & Arup report Here...
Section: 6.2.2 Higher Renewables Penetration, makes interesting reading, as does the analysis of the initial proposal to pump seawater. This turns out to be non-viable in this location for environmental and engineering reasons.
It also turns out that, in addition to the obvious competition from batteries, the interlinks to Victoria and now New South Wales (Read More...) are significant competitors and that the very long life-expectancy of pump-storage makes it less attractive to commercial investors. Batteries are actually more attractive because of their predictably shorter life-expectancy.
And if we use Prof Andrew Blakers numbers, pumped hydro would have an upfront cost of three to five times that of batteries.
Maybe the price equation has changed but again, the Snowy, even with a modest expansion, is far too small to secure energy security if we persist in a reliance on wind energy and solar alone to replace existing coal fired electricity generation. As the above report makes clear, thermal energy will still be required as part of any practical mix. Carbon-based or nuclear?
South Australia is a small part of the National Electricity Market (5.8% of the NEM) and this project might have made a contribution but we would need several Snowy Schemes to do the same for the larger states. Good luck with the Greens on that one.
Other Mechanical
Pumping water uphill can be viewed as mechanical energy storage. Storing potential energy, as the grandchildren do with the cuckoo-clock in the kitchen, hauling up the pinecone shaped weights to watch them fall while driving the mechanism and, of course, the music box, winding the spring.
It's somewhat ironic that when electric trams replaced cable cars the energy efficiency equation worked the other way. Why convert mechanical energy to electricity and back to mechanical energy when one can use the mechanical energy directly, the cable car companies demanded to know. The same argument applied to factories in which mechanical energy was delivered directly to machines by belts from overhead shafts.
But this is where electricity shines.
Flywheels are a good example of mechanical storage. Motors store energy into flywheels by accelerating their spins to very high rates (up to 50,000 rpm). The motor can later use that stored kinetic energy to generate electricity by going into reverse. Flywheels are commonly left in a vacuum so as to minimize air friction, which would slow the wheel.
The Stephentown Spindle in Stephentown, New York, unveiled in 2011 with a capacity of 20 MW, was the first commercial use of flywheel technology to regulate the grid in the United States. Several other flywheel facilities have since come on line.
Flywheels are not suitable for long-term energy storage, but are very effective for load-levelling and load-shifting applications. Flywheels are known for their long-life cycle, high-energy density, low maintenance costs, and quick response speeds. Over short peaks the latest flywheels have similar energy efficiency to batteries.
Compressed air has also been used overseas (in the US and Germany) as an energy complement to a gas turbine electricity generator. The air is pumped into a cavern when electricity is cheap and boosts the energy of a gas turbine during peak load.
My brother is an advocate of mechanically lifting weights. Others suggest pressurising gas to drive water through a turbine, for example in underwater bags. But in each case, capital cost and conversion efficiency remain significant hurdles.
Heat
Heat has been the principal source of electrical energy for over a century. This has been principally derived from burning fossil fuels but geothermal heat, solar heat, and heat from nuclear fission have also played a significant part in various parts of the world.
As mentioned elsewhere, converting heat to electrical energy is most efficiently done on a small scale using thermo couples. Mechanically, the most efficient conversion is by Sterling Engine. But combining cost and efficiency, using steam or hot gas to spin turbines, and thus alternators, is almost always used in thermal power stations. Energy efficiencies of up to 47% are possible using ultra-critical technology.
In a power station in Spain heat from solar collectors is stored in molten salt that in turn heats water but the efficiency of relatively low temperature steam is well below ultra-critical.
So it makes little sense to store electricity and heat, unless there is another use for low-grade heat, for example water or greenhouse heating, as considerably less than half the energy will be recoverable as electricity.
There is a discussion of the Spanish experience using hot salt for storage, in the article on Spain.
Other electro-chemical conversion possibilities
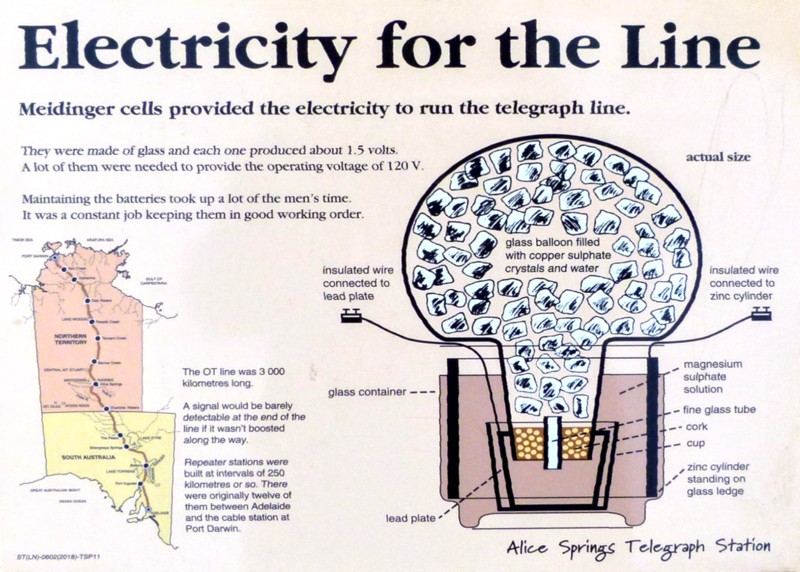
In June 2021, Wendy and I, with our friends Craig and Sonia visited Alice Springs in Central Australia and while there went to see the historic Overland Telegraph (repeater) Station where telegraph messages were weakly received and re-keyed with renewed energy. But where did the energy come from?
It was from batteries of course but they had no way to charge these batteries and they were primary cells, that consumed the chemicals that combined to release their electricity. Tons of chemicals were shipped in by camel and tending the batteries was a principal responsibility of the Station staff taking up much of their time.
The electrical energy that was thus released was chemical energy that had been imparted to the copper-sulphate magnesium-sulphate and zinc metal in furnaces, in factories back in the industrial world, primarily by burning coal.
Similarly, the zinc-carbon or alkaline primary cells in your TV remote consume chemicals created by smelting, often using electricity to impart that energy.
Thus, today, aluminium is sometimes described as frozen electricity. But just like hydrogen far more energy is imparted than can be recovered as electricity.
Nevertheless, the hunt is still on for more efficient electrochemical processes that could supplant conventional batteries.